The revised version of L5 came into effect on 2 December 2013. It contains, as it did previously, the Control of Substances Hazardous to Health Regulations 2002 as amended, printed with accompanying Approved Code of Practice (ACoP) and Guidance.
The document supersedes the fifth edition, which came into force in April 2005.
The ACoP and guidance supporting regulations 7, 9, 10 and 11 has been updated to take account of regulatory changes such as the introduction of the EU Regulations for the Registration, Evaluation, Authorisation and restriction of Chemicals (REACH) Enforcement and Classification, Labelling and Packaging (CLP). It also incorporates various amendments and new regulations introduced since 2005, including the introduction of the Control of Asbestos Regulations 2012.
The revision is part of a wholesale review and update of ACoPs being undertaken to make HSE advice clearer and avoid gold plating.
The main effect of the review has been to modernise the language used, update references to other legislation and remove “gold plating”, ie. requirements from the ACoP which go beyond that intended by the regulations. The document has also been considerably shortened by referencing additional information and guidance on the HSE website, rather than including the detail within the document itself.
Here are three of the key changes to the guidance. A full summary of all changes is available from the Barbour EHS service.
Regulation 2 – Interpretation
The section on biological agents has been expanded. Guidance has been added which was previously located under Regulation 5. This explains the type of exposure to biological agents to which the COSHH regulations apply — direct work with such agents, and incidental exposure as a result of such work, but not exposure un-related to the work activity, e.g. where one employee catches an infection from another.
There is a new section of guidance on the topic of asthmagens. Notes have been imported from the previous Appendix 3, describing the characteristics of occupational asthma.
Under the heading “Other points to consider” there is a paragraph in similar terms to the previous version that lists factors to consider when deciding if a substance is hazardous. Two additions are made to this list: nanoparticles and wet work (linked to dermatitis).
Regulation 6 – Assessment of the risk to health created by work involving substances hazardous to health
The guidance and ACoP material has been radically changed. Large amounts of guidance have been removed including those parts that went into detail about the role of safety data sheets.
Competency of assessors
As before there is a section, which considers the competency of the assessor. New guidance (in para 52) recommends that employers appointing a person to assist in the assessment or its implementation should put in place a, “formal, written specification for the work that is being planned”.
New guidance has been added on the subject of biological agents in paragraphs 65 and 66. The primary purpose of this is to direct readers to other web based sources of information.
Implementation
A new paragraph of ACoP material has been added (paragraph 71). This simply describes the need to implement the findings of the risk assessment before work proceeds.
It is recommended that a prioritised action plan is produced.
Combined assessment
As before the guidance proposes that employers may choose to combine their assessment under COSHH with a risk assessment produced under the Management of Health and Safety at Work Regulations 1999 or DSEAR 2002. A new phrase has been appended to this advice, “employers should ensure that all assessments are suitable and sufficient to comply with relevant regulations.”
Regulation 7 – Prevention or control of exposure

Paragraphs 99 to 119 provide new guidance on applying the “Principles of good practice for the control of substances hazardous to health“.
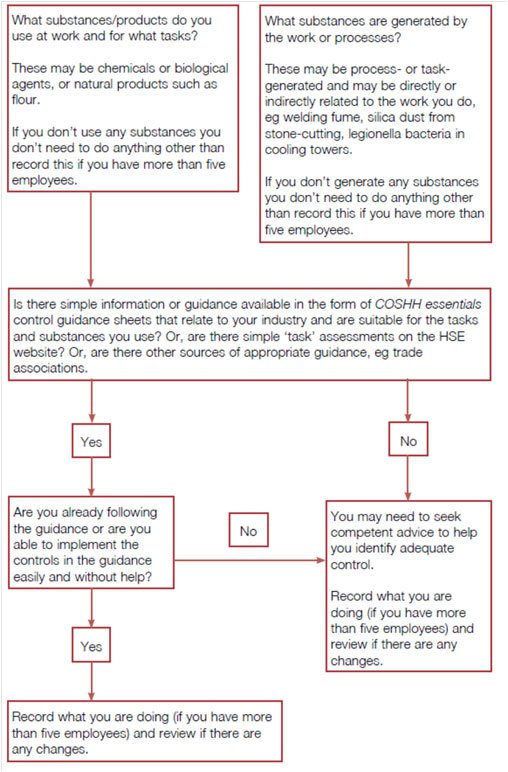
This is followed as before with a “Route map for adequate control”. However, rather than two flow diagrams, one for experts, and one for non-experts, there is now a single route map (above). The guidance has also been re-written.
The shortened guidance on vaccines reminds employers that not all employees will take up the offer, and some may not respond to the vaccine, therefore this is an additional measure rather than the sole source of protection.
Adequate control for exposure by inhalation
Guidance on the subject of workplace exposure limits is unchanged except that one paragraph has been deleted. It had defined two broad groups of substances, which were assigned a WEL.
In addition there was guidance supplying further detail about the test process and the mechanism for achieving a good seal. The guidance now simply states, “More information about fit testing can be found at www.hse.gov.uk/respiratory-protective-equipment.“
The Safety Conversation Podcast: Listen now!
The Safety Conversation with SHP (previously the Safety and Health Podcast) aims to bring you the latest news, insights and legislation updates in the form of interviews, discussions and panel debates from leading figures within the profession.
Find us on Apple Podcasts, Spotify and Google Podcasts, subscribe and join the conversation today!